Week 10
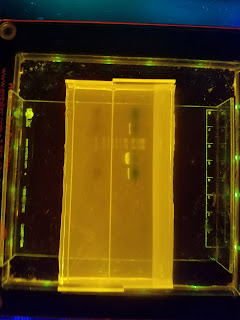
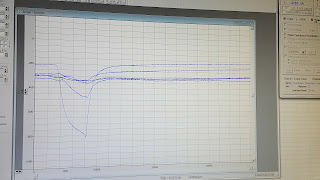
This week marked the conclusion of my Senior Project and thus my time at BNI. In retrospect, Week 1 seems like an eternity ago, but the project itself went by unbelievably quickly. My hypothesis about the primers was correct, as I ran a gel on Friday's PCR products and finally found success on Monday, which meant I could resume the transcription process. On Tuesday, I did exactly that and also attended a seminar about ongoing research in spinocerebellar ataxia type 1 (SCA1). On Wednesday, I ran a gel to verify the RNA products of the transcription process, but still didn't get anything beyond the one type last week (of four desired types). If I had more time, I would definitely try again next week. Lastly, I spent Friday training and instructing another high school student who will be conducting research in Dr. Chang's lab over the summer. All in all, the entire experience was fantastic and a worthwhile exposure to research. If you want to hear more of my thoughts